The ammonia molecule remains at the center of growing agricultural production through fertilizers and embracing renewable energy worldwide. The gist of increased agricultural yield results from advancements in ammonia production and distribution across the channels.
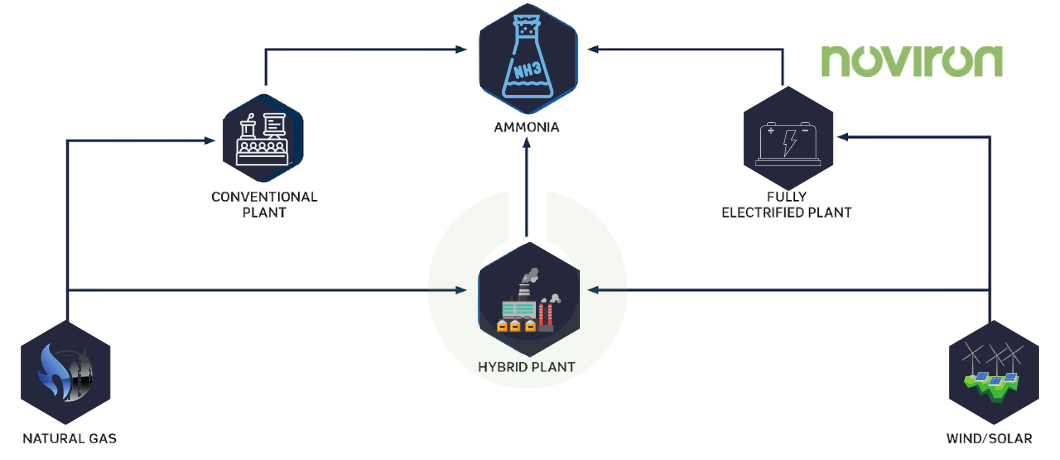
Beginning in the early 20th century, the Haber-Bosch (HB) process for synthesizing ammonia, converted to nitrogen fertilizers or N fertilizers, has become very popular. The traditional HB process uses methane [CH4] as an energy source and feedstock or fuel source of hydrogen to combine with nitrogen from the air to produce ammonia. This sort of ammonia is called brown or grey ammonia.
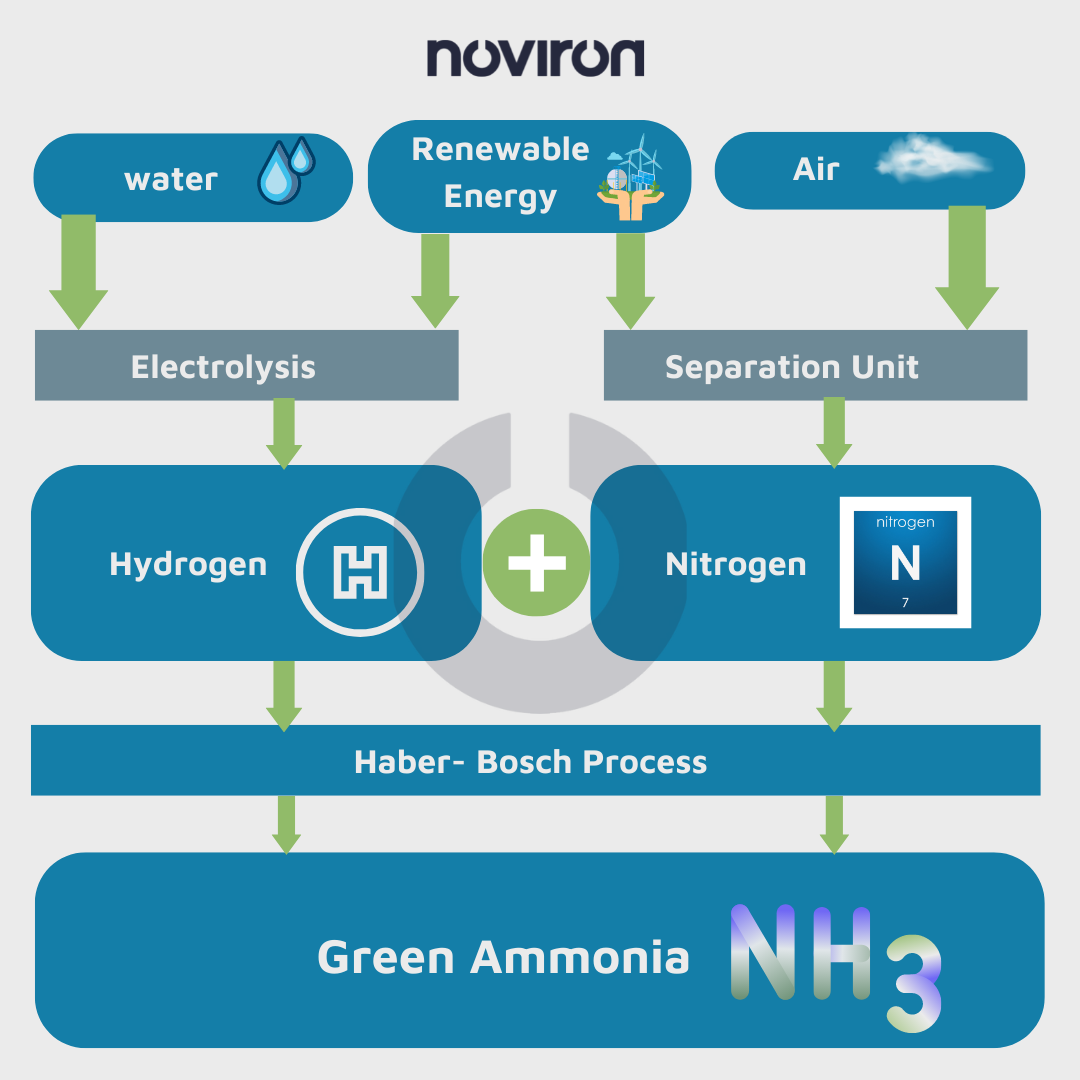
The electrically driven HB process has recently become a focus of interest because of its ability to be combined with renewable electricity via green ammonia. In this method, hydrogen is separated from water by electrolysis, and nitrogen is separated from the air by pressure swing adsorption (PSA) [is a technical way of differentiating a gaseous material from a combination of gases (such as air) under pressure according to the molecular characteristics of the gas and association with the adsorbent material], allowing renewable energy to be used to produce N fertilizer.
In addition, ammonia can be used as a hydrogen carrier for dense liquid energy storage and as a pipeline to supply renewable energy to the local electric grid. In this way, it becomes an ideal solution for the Haber-Bosch electric process.
CO2 emissions, Green ammonia, and the Net Zero goals
Ammonia is the initial step in all composts with mineral nitrogen, providing a scaffold between the nitrogen in the atmosphere and the food we eat.
However, the basic form that ammonia provides contains several significant downsides for the global environment. Ammonia production has about 2% of total final energy consumption, almost all from fossil fuels, resulting in a carbon dioxide (CO2) footprint equivalent to South Africa’s total energy system emissions.
Timur Gul, Head of the Energy Technology Policy Division IEA, says, “The world will need more ammonia but cannot afford the emissions that come with its production.”
True carbon neutrality requires that at no point in the supply chain any greenhouse gas is released: ammonia and hydrogen production, carrier synthesis, fuel transportation, pipeline or truck distribution, and dehydrogenation should be completely carbon-free. The carbon source should be simple air capture or biomass for producing hydrocarbons.
Green ammonia fuel as renewable energy
Before understanding green ammonia, let’s first get acquainted with green hydrogen. Three-quarters of the sun is hydrogen, making it the most plentiful component in the world. This means that this smallest molecule has a massive amount of energy, being the most negligible mass. There is a typical method called steam reforming of methane, which produces hydrogen or natural gas, and the only drawback is that a lot of carbon dioxide is made in this process. So we proportionately generate a carbon footprint by producing hydrogen using conventional resources.
To suppress this carbon footprint, we have another way to produce hydrogen through electrolysis; by using electricity, we split oxygen and hydrogen from water. When green electricity, i.e., electricity from renewable sources, is used to produce hydrogen, the type of H2 made is called green hydrogen as there is no carbon footprint in its production.
Now let’s understand the concept behind green ammonia. There is a strong connection between ammonia and hydrogen. Namely, ammonia is the most important derivative of hydrogen. At the same time, ammonia is much easier to handle and transport than hydrogen. This means that storing hydrogen needs to be compressed and condensed, which involves a lot of cost and maintenance.
Green hydrogen is produced from renewable energy sources, which is later used to produce ammonia as a fuel or hydrogen energy vector; called green ammonia. On the other hand, ammonia is a widely used industrial chemical ingredient. It has been used as an effective fertilizer for decades and established logistics worldwide. In addition, NH3 or ammonia has another broad potential – with sustainable production, it can become an emission-free green fuel or be used as a storage and energy carrier. This kind of ammonia is also emission-free in terms of its carbon footprint.
Industries have developed technologies and catalysts for the production of green ammonia from renewables, including solar, wind, water, and air. With a documented experience in the industry and a deep understanding of the science behind ammonia, it is widely used as a core component of the hydrogen economy.
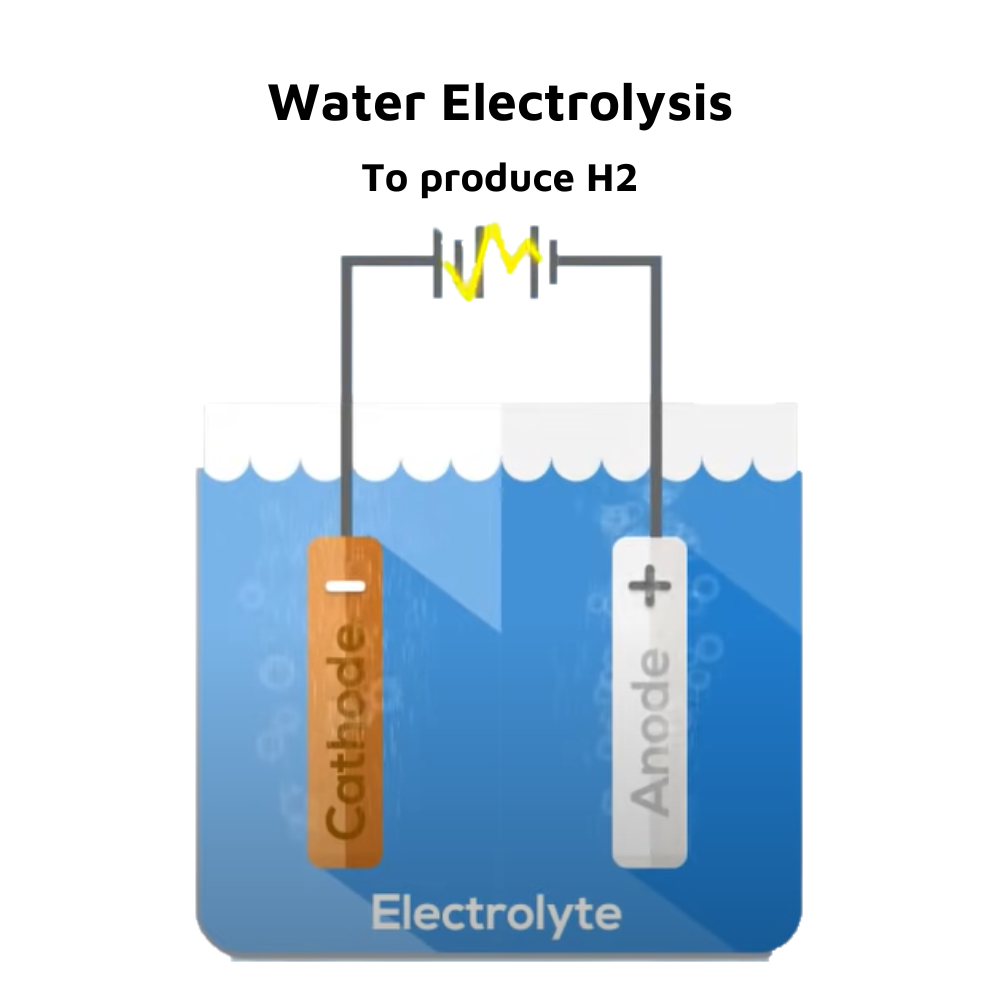
Water electrolysis and methane pyrolysis
Water Electrolysis involves splitting water into hydrogen [H2] and oxygen [O2] using electricity. When direct current is supplied by water, oxygen appears at the anode [+ve], while hydrogen is released from the cathode [-ve]. In fact, by volume, the amount of hydrogen produced is twice that of oxygen. Since pure water is not a superconductor of electricity, salt is added to it to make it a conductive material. Naturally, this is not a very environmentally friendly procedure. If a proton exchange membrane [PEM] or polymer-electrolyte membrane [PEM] is used, it is a semi-permeable membrane that allows H2 and O2 atoms to pass through. The PEM is usually made of ionomers and prepared to conduct protons, acting as both an electronic insulator and a reaction barrier for oxygen and hydrogen. However, PEM is an expensive method since it includes platinum and gold in the membrane as active ingredients. This is further remedied with Anion Exchange Membrane [AEM], which uses an alkaline solid polymer membrane that is more cost-effective and produces compressed hydrogen directly. Thus, AEM is useful in using renewable energy as a source of electricity, low cost, generating directly compressed hydrogen, high purity, and a more straightforward solution.
source: https://www.phisueahouse.com/
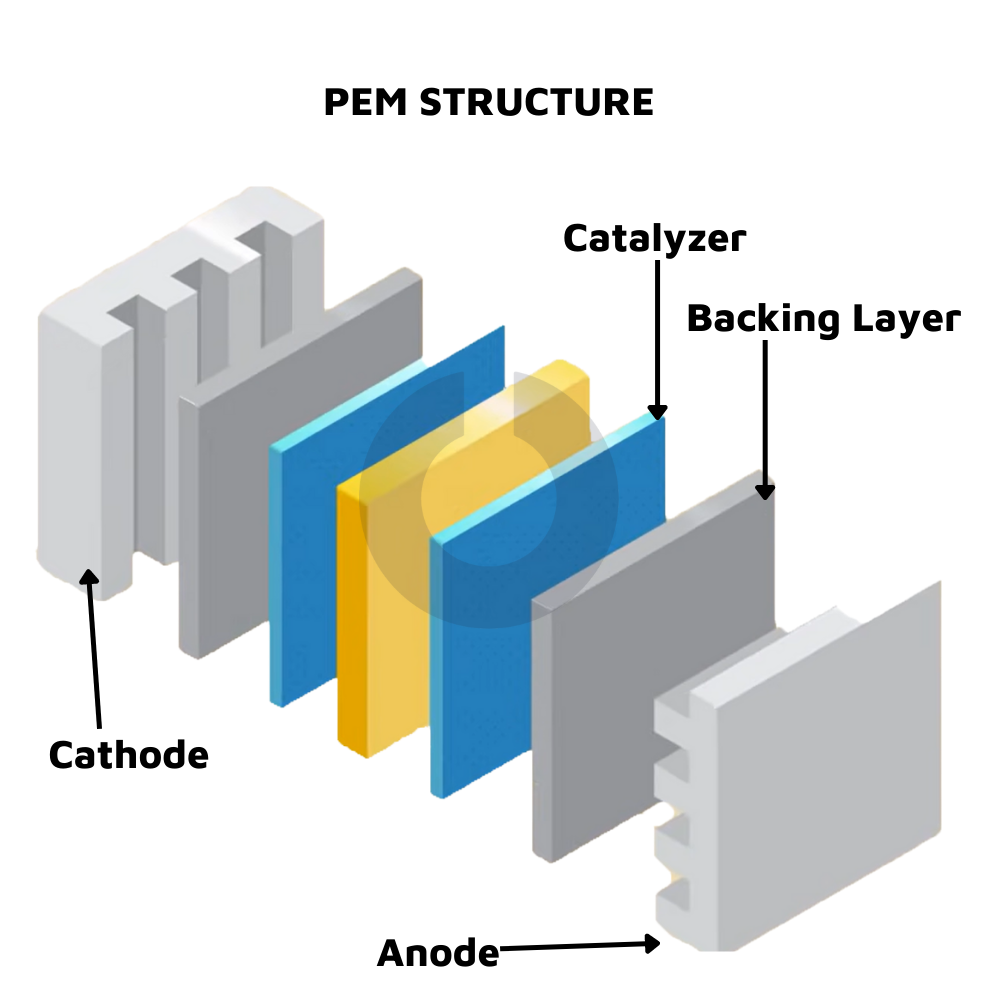
source: https://www.phisueahouse.com/
Methane pyrolysis – is a zero-carbon H2 production method considered an environmentally friendly process to overcome renewable energy fluctuations. It enables the production of hydrogen and carbon from methane. In this operation, methane [CH4] is heated to more than 1,000 degrees Celsius using renewable electricity. The methane is then split into the hot reactor center, forming hydrogen gas and solid carbon. The hydrogen rises to the top. This process does not produce carbon dioxide gas, and the carbon remains at the end as a solid pellet. The whole process is CO2-free as it uses renewable electricity from the beginning, and there are no carbon emissions at the end due to the carbon pellets. Hydrogen can now be used as an energy carrier in the industry, transportation, or households. The solid carbon can be further processed or stored.
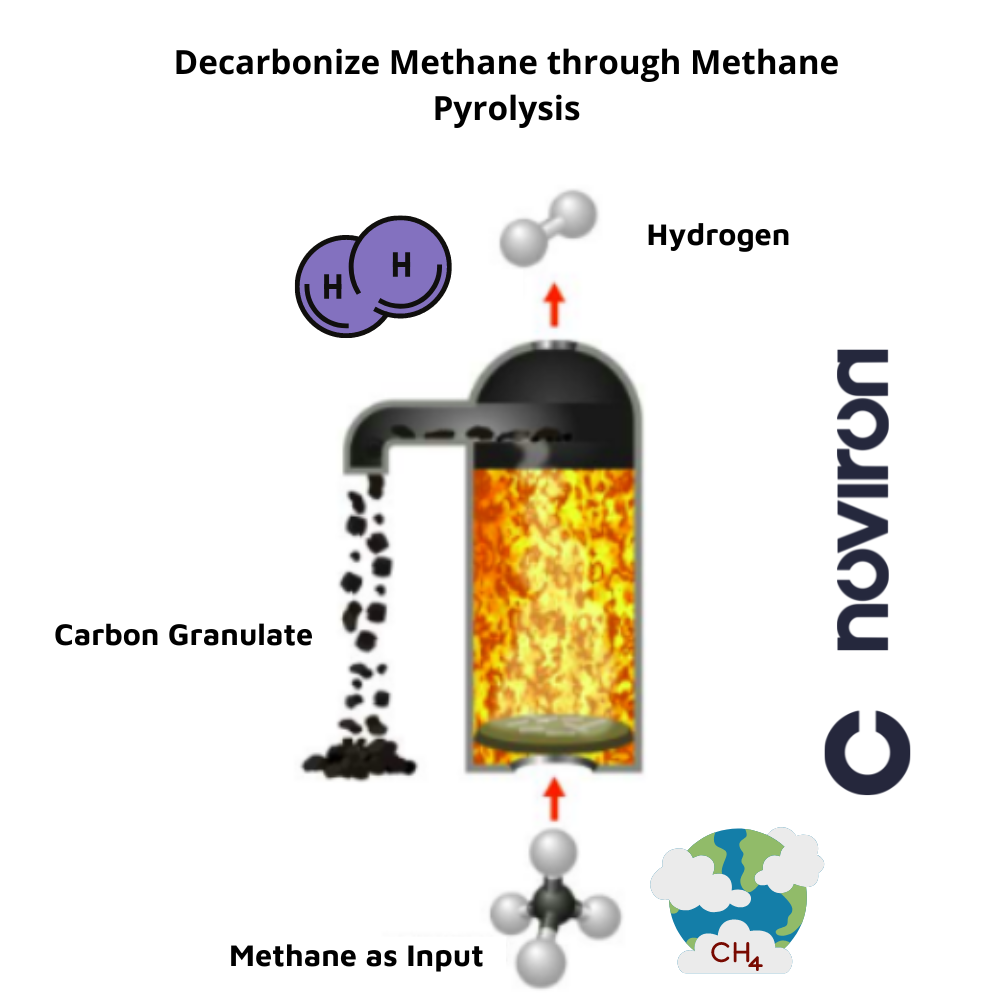
source: arpa-e
Green or blue Ammonia: Which one is better?
Blue Colored
Blue ammonia is a form of conventional ammonia with CO2 capture and storage byproduct, which attenuates the climate impact compared to gray or carbonized ammonia. Here, blue is the color of hydrogen produced from fossil fuels, but with carbon capture and storage. The International Energy Agency technically uses CO2 capture as well as use or storage, “CCUS.” But carbon dioxide is only used and stored in oil recovery operations, and it’s still a big question about achieving sustainability in this sector.
From the production of H2 and NH3, CO2 is used in an in-combined ammonia/urea unit to produce urea from ammonia – and released into the air soon after the urea is used in agricultural processes. Most other uses of carbon dioxide involve the production of hydrocarbons, starting with methane gas.
Many fertilizer manufacturers have come up with such projects in recent years. Blue ammonia is debatable and requires industry protocols. For example, using CO2 for enhanced oil recovery is less valuable for the environment than releasing it permanently into the ground. Nor is it acceptable to deal effectively with climate conditions, thereby achieving net-zero goals.
Green Ammonia
Did you know? Producing 1 ton of conventional or brown ammonia releases two tons of carbon dioxide. Countries worldwide are working to achieve a net zero or zero carbon footprint, and traditional ammonia is a significant obstacle to achieving environmental and sustainability goals. Producing green ammonia from zero-carbon renewable energy sources would help to reach these goals.
Green ammonia production uses renewable energy sources such as hydropower, solar power, or wind turbines. Unlike gray and blue ammonia, which are produced with fossil fuels as a feedstock or have a carbon footprint at their core, the feedstock for green ammonia production is hydrogen obtained by electrolysis of water, a process powered by renewable energy sources, and nitrogen obtained from the air using an air separation unit. Green ammonia can then be synthesized from nitrogen and hydrogen by various techniques, with the Haber-Bosch process being the only method currently used commercially.
Why Green Ammonia?
Like fossil or non-renewable fuels, ammonia breaks its chemical bonds, releasing both a carrier of chemical energy and a potential fuel. Typically, ammonia does not emit carbon dioxide when used as a fuel, and its environmental performance can be even more outstanding if sustainable energy is used to produce it.
Ammonia is the world’s second most widely used chemical, with an annual production of more than 180 million tons, of which 20 million tons are produced in the EU and about 20 million tons sold annually. Ammonia is mainly used in agricultural products, a sector that is receiving increasing attention for its environmental impact, with 80% of the world’s ammonia production going to fertilizers and 20% to industrial products.
Nature has endowed ammonia with characteristics that seem to make it an ideal ingredient for the future hydrogen economy. Here are some of the conventional and advance uses of Ammonia.
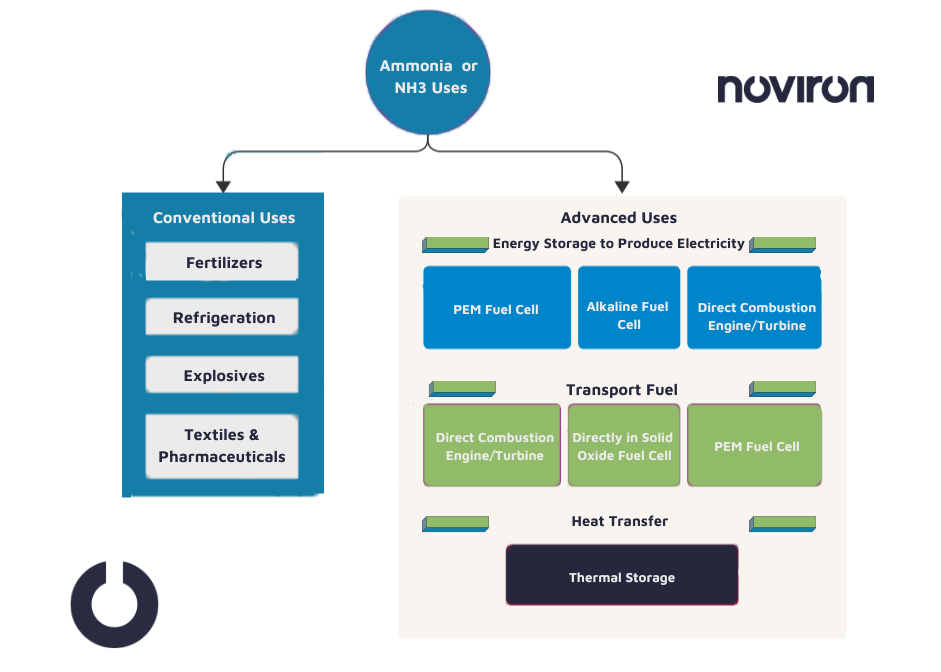
Conclusion
Some chemical properties distinguish it from other available energy alternatives. Ammonia or NH3 has a higher energy density (12.7 MJ/L) than liquid H2 (8.5 MJ/L). Liquid H2 must be stored under cryogenic (super-low storage temperature) conditions of -253 °C. In contrast, ammonia can be stored at a much less energy-intensive and relatively higher temperature than hydrogen, i.e., at -33 °C. Ammonia, while dangerous to handle, is much less flammable than hydrogen and has ten times the energy density of a lithium-ion battery. Thus, energy storage is a viable option for green NH3.
In addition, thanks to a century of ammonia used in agriculture, there is already an extensive ammonia infrastructure. About 180 million metric tons (t) of ammonia are produced annually worldwide, and 120 ports are prepared for ammonia terminals. Thus, the energy produced using green ammonia is a win-win, as it is the future of energy and the energy of the future.
Ammonia is presently one of the primary energizers being viewed by the marine sector too to empower the shipping niche to meet new CO2 emission goals proposed by 2030 and 2050. Ammonia is likewise considered a way to store sustainable power for various use cases and a transporter for hydrogen energy vector transportation. Worldwide ammonia production, as mentioned above, stands at 180mn t/yr. Yet, its expected use as an energy source and energy transporter could see interest for it climb to a multi-billion ton market for use in the scope of mass application.
Noviron is conducting a LIVE training titled “Green Ammonia Live Training” You can get more details here https://noviron.com/live-online-training-green-ammonia/
Sources:
https://www.sciencedirect.com/science/article/pii/S2590332220306618#bib16
https://cen.acs.org/business/petrochemicals/ammonia-fuel-future/99/i8
